
RUCONEST® is indicated for the treatment of acute angioedema attacks in adults, adolescents, and children (aged 2 years and above) with hereditary angioedema (HAE) due to C1 esterase inhibitor deficiency.
RUCONEST® is the only recombinant human C1 esterase inhibitor (C1-INH) treatment available that targets the fundamental cause of HAE by replacing the missing/dysfunctional C1-INH¹
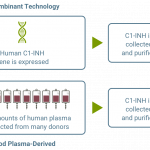
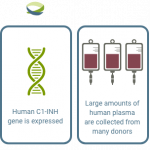
C1-INH: C1 esterase inhibitor.
Production is unaffected by shortages in plasma donations. No risk of human blood-borne virus transmission⁴
A vial of RUCONEST® has a shelf life of 4 years which is longer than other HAE treatments1,2,5-8
No risk of human blood-borne virus transmission4
C1-INH neutralising antibodies are not detected throughout treatment9-12
RUCONEST® provides an optimum dose based on body weight.²
Dosing by body weight²
|
Body weight |
RUCONEST® dose for IV injection |
Volume (mL) of reconstituted solution (150IU/mL) to be administered |
|---|---|---|
|
<84 kg (185lb) |
50 U per kg |
Body weight in kg divided by 3 |
|
≥84 kg (185lb) |
4200 U (2 vials) |
28 mL |
Upon recognition of an HAE attack, RUCONEST® can be administered by slow intravenous (IV) injection for 5 minutes. Patients may self-administer RUCONEST® after receiving appropriate training by a qualified healthcare professional.²
The animation below shows how a patient can prepare and administer a dose of RUCONEST® at home. Before preparing RUCONEST® for injection, read the full instructions in the prescribing information. For more resources on IV administration patient materials, and videos click here. Pharming also has nurse support services which are available for patients in the UK, Netherlands, and Bulgaria.
Treatment preparation animation
Animated guide on preparing and administering RUCONEST® treatment
Treatment preparation video
Instructions for the preparation and self-administration of RUCONEST®
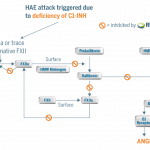
B receptor: bradykinin receptor; C1-INH: C1 esterase; FXII: factor 12; FXIIa: factor 12a; HMW: high molecular weight.
References