Real world
Real world evidence demonstrates that RUCONEST® provides sustained relief for all HAE attacks in just one dose in multiple locations8*

RUCONEST® provides fast, effective, sustained symptom relief with just one dose1-4
FAST
Time to start of symptom relief was <2 hours (median)* in clinical trials, even in the case of severe attacks and regardless of their location1-3
EFFECTIVE
96% of attacks were treated with just one dose,** even in the case of severe attacks and regardless of their location (215/224 attacks in 44 patients)1
SUSTAINED
For at least 3 DAYS n=68 (259 of a total of 280 attacks)†4‡
One dose of RUCONEST® stopped 93% of attacks (259/280 in 68 patients) for at least 3 days4
*Confirmed by the consensus of HAE experts at Pharming Advisory Board meeting on 7th March 2022. **1 or 2 vials depending on the patient weight. †Dosing of RUCONEST® is weight dependent and one dose is, therefore, based on patient weight as follows; <42kg = up to 1 vial, 42-84kg = 50 U/kg (up to 2 vials), >84kg = 2 vials;
‡RUCONEST® is indicated for treatment of acute angioedema attacks in adults, adolescents, and children (aged 2 years and above) with hereditary angioedema (HAE) due to C1 esterase inhibitor deficiency.5
C1-INH: C1 esterase inhibitor; HAE: hereditary angioedema.
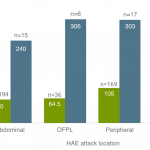
HAE: Hereditary Angioedema; OFPL, oro-facial pharyngeal-laryngeal.
Note: Results are obtained from pooled analysis of data from studies C1 1205 and C1 1310. Adapted from Baker et al., 2018.⁶
*Attacks with 72 hour, post dose follow up data. Sustained response defined as a response (≥20 mm decrease of VAS scores at 2 consecutive time points for 4 hours post treatment) that was not associated with an increase of ≥20 mm or onset of new attack symptoms within 72 hours post dose6. RUCONEST® is indicated for treatment of acute angioedema attacks in adults, adolescents, and children (aged 2 years and above) with hereditary angioedema (HAE) due to C1 esterase inhibitor deficiency.5
Real world evidence demonstrates that RUCONEST® provides sustained relief for all HAE attacks in just one dose in multiple locations8*
For a European patient registry involving:
71 patients
9 countries
2,351 HAE attacks
99.8% of attacks were resolved in just one dose of RUCONEST® regardless of attack location.8
|
All patients (N=71) |
All attacks |
First attack |
Single location |
Two locations |
Three locations |
|---|---|---|---|---|---|
|
Dose |
3,307 |
3,107 |
3,336 |
2,749 |
2,520 |
|
Number of doses, n (%) One |
2,351 (99.8) |
70 (98.6) |
2,239 (99.9) |
107 (98.2) |
5 (100) |
*RUCONEST® is indicated for the treatment of acute angioedema attacks in adults, adolescents and children (aged 2 years and above) with hereditary angioedema
(HAE) due to C1 esterase inhibitor deficiency.5
References